inSafe
inSafe provides complete protection
from the beginning of the medical procedure, through to the disposal of the needle. The specially designed syringe protects the needle at ALL times and its partnering sharps container removes the contaminated needle in complete safety!

Needle protected by a lockable sleeve
Works with all needle types
Easy to see aspiration
Anaesthetic cartridge changeable during procedure
Autoclavable syringe made from metal and plastic. Feels and weighs like a conventional syringe
One handed disposal of contaminated needle
Economical system with low costs per use
Fits 150 needles
Base can be secured to worktop (Cabinet and wall mounted brackets are also available)
Minimal disposable components
inSafe – Safety Syring System Demo Video
How to use inSafe
inSafe provides comprehensive protection from the beginning of the procedure to the disposal of the needle. The specially designed syringe protects the needle at all times and its partnering sharps container completely removes the needles safely without contact.
Legislation and good practise technique dictate that appropriate safety systems are used in dental practices. inSafe is the Best Practise option offering total protection from needlestick injury with full choice of needles and aspiration techniques.
Push on needle adaptor
Screw on needle
Load cartridge
Cover needle
Remove needle safely
Twist and
Withdraw syringe
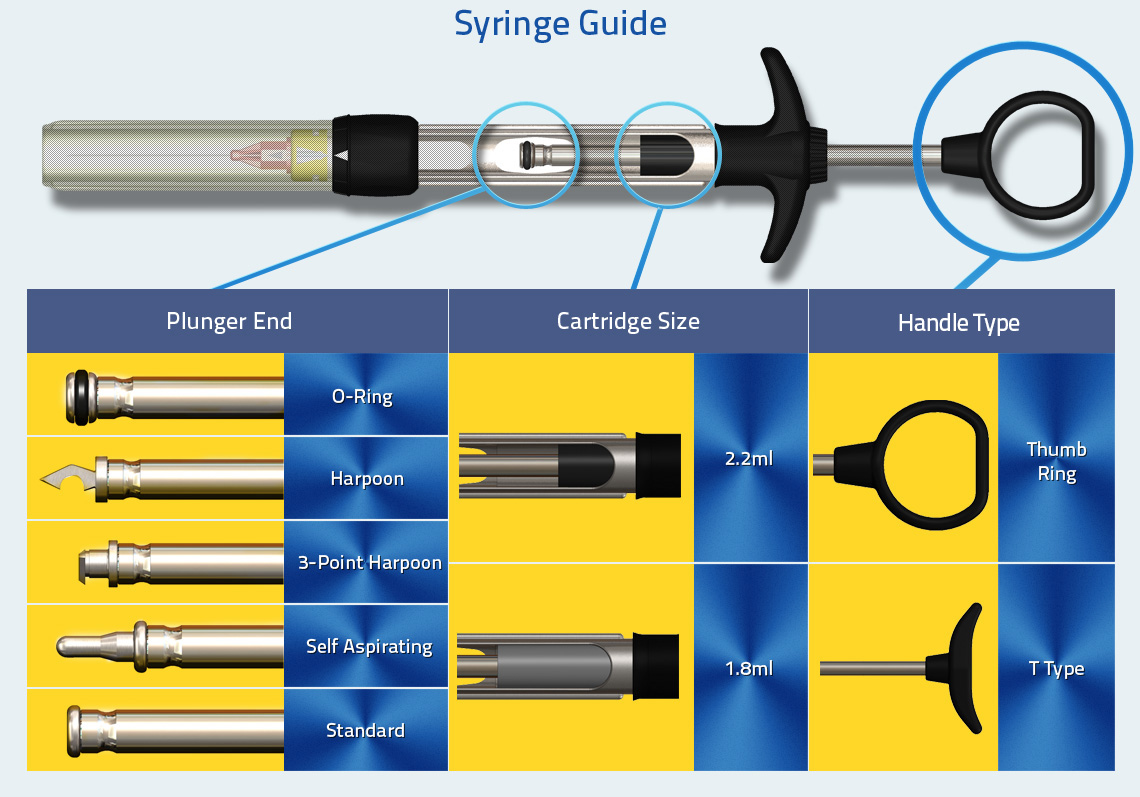
Syringe Guide
inSafe Syringe Guide
A syringe to suit the way you work
With two handle types, two cartridge types and sizes, and with a choice of five aspiration options, inSafe can be used with all types of needle, cartridge and every aspiration technique.
Need help finding the right configuration? Try our Order Assitant to find the product matching your needs.
Container Mounting Options
inSafe Container Mounting Options



Reprocessing
General
Process equipment (e.g. Washer-disinfectors and Sterilisers) must be subjected to periodic testing and validation in accordance with a recognised standard/reference document e.g. ISO 15883, CFPP01-01.
Process equipment must be operated in accordance with the manufacturer’s instructions.
Ensure appropriate Personnel Protective Equipment (PPE) is worn at all times when carrying out decontamination activities.
Cleaning
The device can be cleaned either by using an automated process or manually cleaned by hand.
A validated automated washer-disinfector is preferable to manual cleaning as it provides thermal disinfection and a reproducible process that should give consistently reliable results. However, the instructions below provide details of how the device can be processed using either method.
Detergents should be CE marked and specifically intended for cleaning medical devices.
Automated cleaning
- Ensure any movable parts of the device are opened where possible in order to facilitate the cleaning process. To do so, lock the InSafe syringe sleeve in the forward position. Unscrew the plunger retaining nut and separate the plunger assembly from the body of the device (as shown in image above). The plunger should remain detached from the body assembly during the cleaning process as this assists the cleaning process.
- Both the handle and body assembly can be processed in an automated washer-disinfector.
- Do not overload the washer disinfector as this may compromise the effectiveness of the process.
- Process using a standard instrument cycle.
- After processing, the device must be visually inspected to ensure it is clean and dry. If there is any evidence that the device is not clean it must be reprocessed.
- These instructions have been validated using the following washer-disinfector programmes:
- Programme 1
Cold pre-wash
Wash stage: 5 minutes using an alkaline detergent with a pH 13-14.
Rinse stage: 1 minute
Disinfection stage: 90°C for a minimum 1 minute / Ao Value 600
Drying - Programme 2
Cold pre-wash: 2 minutes
Wash stage: 2 minutes using a neutral detergent
Rinse stage: 2 minutes
Disinfection stage: 80°C for a minimum 10 minute / Ao Value 600
Drying: 90°C - Ultrasonic Bath
If a suitable automated washer-disinfector is not available the device can be cleaned in an ultrasonic bath, but it should be manually cleaned, in accordance with the instructions (right), prior to being placed in the bath. When removed from the bath the device must be rinsed thoroughly in clean (preferably purified) water.
Manual cleaning
- Fully immerse the device in a solution of detergent and water. The ratio of detergent water should be consistent with that recommended by the detergent manufacturer*.
- Fully immerse the device throughout the cleaning process to prevent splashing and the creation of aerosols.
- Ensure the temperature of the water used for the initial immersion of the device does not exceed 45°C.
- Using a low-linting disposable cloth wipe all the accessible surfaces of the device. If necessary, a soft nylon brush may also be used. Agitate and irrigate the device as appropriate to help dislodge any contamination.
- Rinse in clean (preferably purified) water.
- Dry the device using a lint free single-use cloth.
- Visually inspect the device to ensure it is thoroughly clean. Repeat the cleaning process if necessary.
- *This process was validated using an enzymatic detergent.
Sterilisation
- Sterilise using moist heat (steam).
- Ensure the InSafe Syringe sleeve is locked in the forward position and that the plunger assembly is detached from the body assembly during sterilization.
- If the autoclave is a vacuum steriliser, the plunger and body assembly can be placed together in an autoclavable bag and sealed prior to processing. If the autoclave is a Type N benchtop (gravity displacement) steriliser the device must not be wrapped prior to the sterilization process as this will inhibit steam penetration.
- These instructions have been validated using a porous load steam steriliser (Autoclave) operating on a 134°C to 137°C cycle for a minimum 3 minutes.
The Facts about needlestick Injuries: Did you know..?
The EU Directive for the Prevention of Sharps Injuries in the Hospital and Healthcare Sector:
In June 2010, a new European Directive was published on sharps injuries with the aim of creating the safest possible working environment by preventing injuries to workers at risk. All EU Member states are required to bring into force national legislation or legally binding agreements to implement the Directive.
What does the Directive say?
Risk of needlestick injury must be controlled by measures including the following:
- Safe Procedures – specifying and implementing safe procedures for using and disposing of sharp medical instruments and contaminated waste. The practice of recapping shall be banned with immediate effect. These procedures shall be regularly reassessed and shall form an integral part of the measures for the information and training of workers
- Engineering Controls – providing medical devices incorporating safety engineered protection mechanisms
There are a number of selection criteria to be considered when considering a safety – engineered medical device.
What does this mean for me?
The Directives state where risk cannot be eliminated the employer shall take appropriate measures to minimise the risks. Appropriate measures to minimise the risks would include the provision by employers of safer needle devices and sharps containers.
As a minimum requirement, the Directives state: „Where there is a risk of mechanical contact with moving parts of work equipment which could lead to accidents, those parts must be provided with guards or devices to prevent access to danger zones or to halt movement of dangerous parts before danger zones are reached“
How does inSafe measure up?
There are a number of factors to consider when choosing a safer sharp:
Factors of Consideration
inSafe – Safer Sharp System
Do you have any questions?
We’re here for you!
Want to find out more about our products?
Schedule a consultation with our product experts or send us your request in advance using our contact form.